On October 14, 2025, Jacob S. Brenner from the University of Pennsylvania published a study in the Journal of Neuroinflammation titled “Targeted lipid nanoparticles containing IL-10 mRNA improve outcomes in experimental intracerebral hemorrhage,” revealing that lipid nanoparticles targeting the delivery of IL-10 mRNA improve outcomes in experimental intracerebral hemorrhage.

Intracerebral hemorrhage (ICH) is a type of stroke with high mortality and disability rates, and there is currently a lack of specific treatments. The inflammatory response following ICH leads to secondary injury, and the inflamed cerebrovascular endothelium serves as a critical gateway for peripheral immune cells to enter the brain, making it a potential therapeutic target. However, systemic anti-inflammatory therapies are limited due to side effects or low delivery efficiency to the brain. This study hypothesized that targeted delivery of mRNA encoding the anti-inflammatory cytokine IL-10 to cerebral blood vessels could improve outcomes in a mouse model of ICH. The authors utilized microfluidic technology to prepare lipid nanoparticles (LNPs) encapsulating IL-10 mRNA and conjugated with antibodies targeting vascular cell adhesion molecule (VCAM), achieving specific delivery to the inflamed cerebrovasculature. VCAM-LNPs increased brain delivery efficiency by 4-10 times, significantly reduced hematoma size and lesion volume, improved motor function, and acted potentially by modulating macrophages. This study proposes a novel targeted delivery strategy offering new hope for ICH treatment.
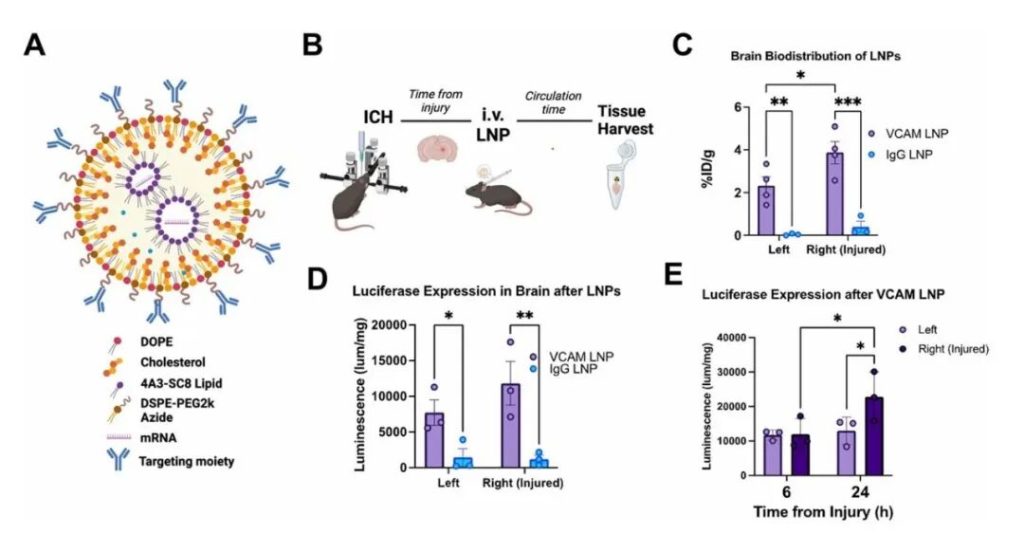
Figure 1: VCAM-Targeted LNPs Show Superior Brain Delivery and Expression Compared to Non-Targeted Controls in ICH Mice
The authors had previously found that VCAM-targeted liposomes delivered more efficiently to the brain in ICH mice than non-targeted controls, remaining effective even with increased vascular permeability. However, intravenous LNP injection can trigger significant immune responses, affecting delivery efficiency. Prior studies relied on commercial LNPs, whose composition is proprietary, making modification difficult. Therefore, the authors redesigned VCAM-targeted LNPs using the ionizable lipid 4A3-SC8, which showed superior expression efficiency compared to commercial lipids. The new LNPs were comparable in size, conjugation efficiency, and stability to previous liposomes and commercial LNPs. ICH mice tolerated them well.
First, an ICH model was established by intrastriatal collagenase injection. Mice received intravenous injections of radiolabeled VCAM-LNPs or IgG-LNP controls 4 hours post-injury, circulated for 30 minutes, and were then sampled. Results: Radiolabeled tracing showed that VCAM-LNPs accumulated approximately 10 times more in the brain than controls, primarily in the injured hemisphere. Spleen distribution was also higher. Brain VCAM signal decreased over time. VCAM-LNPs carrying luciferase mRNA showed significantly higher brain expression than controls, and the signal in the injured hemisphere intensified over time. The newly developed VCAM-LNPs efficiently targeted ICH brain tissue, significantly enhancing mRNA expression in the injured brain.
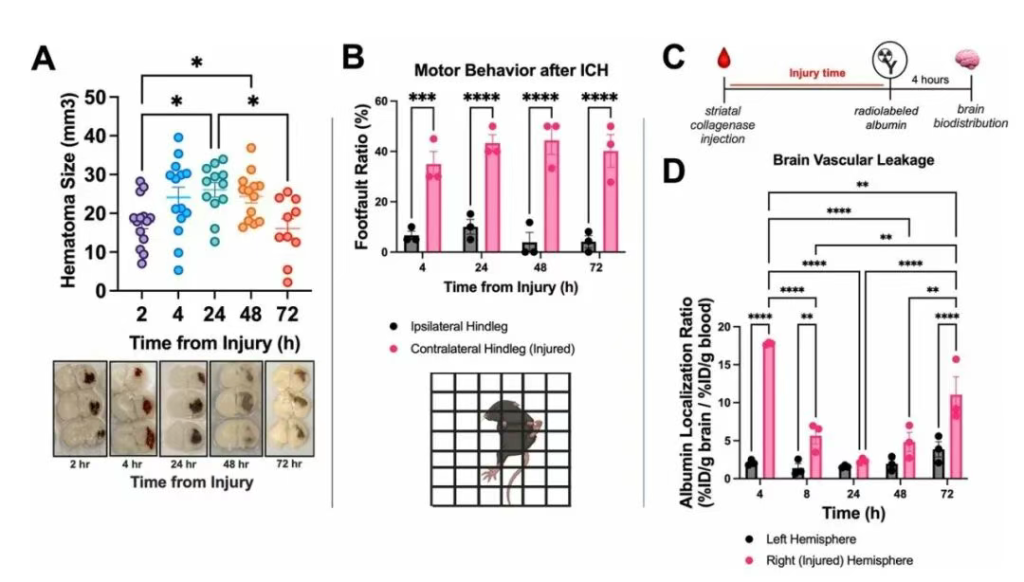
Figure 2: Establishment of a High-Throughput Model for Screening Potential ICH Therapeutics
Intrastriatal collagenase injection is a gold standard method for experimental ICH in mice. Compared to other cerebrovascular injury models, this procedure is simple, produces predictable phenotypes and high survival rates by adjusting collagenase dose, facilitating the establishment of a disease model for drug screening. The authors previously found that hematomas continuously expanded within 24 hours in this model and remained stable for 48 hours. Results: This study further found that hematomas began to shrink at 72 hours. Concurrently, mice developed contralateral limb hemiparesis, which remained stable during this period. The authors had previously observed radiolabeled albumin leakage into brain tissue within hours post-ICH in the collagenase model, disappearing after 24 hours. Extending observation to 72 hours, this study discovered a second wave of albumin leakage in the injured hemisphere, potentially reflecting the inflammatory response post-ICH and representing a potential therapeutic target. Hematoma size, motor function, and vascular leakage constitute a high-throughput evaluation system for screening effective LNP therapies.
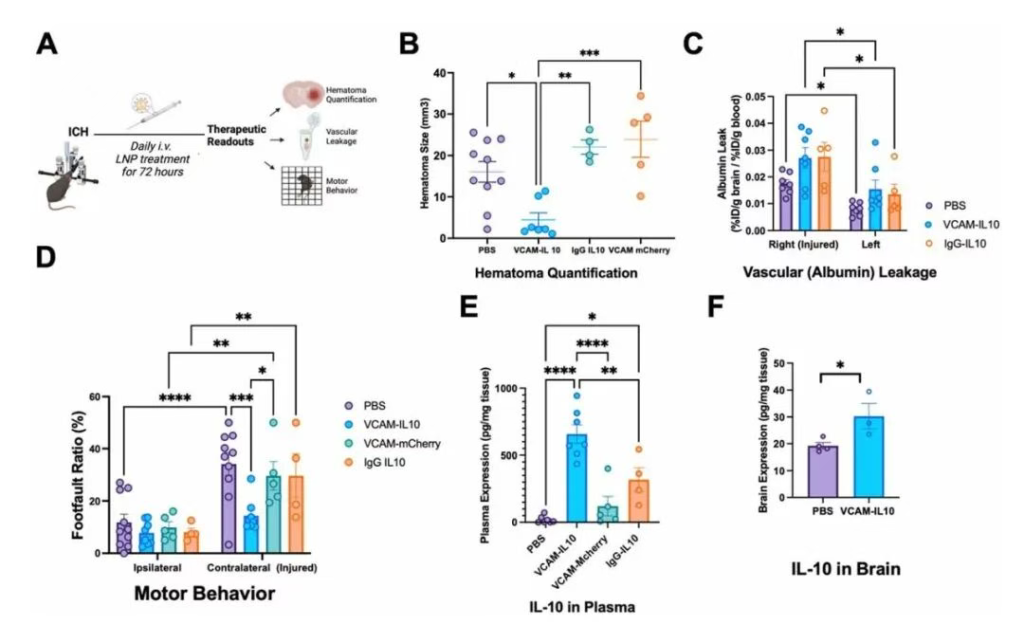
Figure 3: VCAM-IL10-LNP Treatment Reduces Hematoma Volume and Improves Behavioral Function
In the established ICH model, the authors evaluated the therapeutic effect of VCAM-targeted LNPs carrying IL-10 mRNA (VCAM-IL10-LNPs). Mice received treatment at 0.5, 24, 48, and 68 hours post-ICH, with endpoints assessed at 72 hours. Results: Compared to the control group, VCAM-IL10-LNP treatment significantly reduced hematoma volume and markedly improved motor function, accompanied by higher IL-10 expression levels in both plasma and brain tissue. Although the treatment did not reduce vascular leakage at 72 hours, dynamic MRI monitoring showed no difference in hematoma size at 24 hours between groups, with subsequent more significant lesion reduction in the VCAM-IL10-LNP group, suggesting it may function by promoting hematoma resolution rather than inhibiting initial bleeding. In summary, VCAM-IL10-LNPs effectively reduce brain injury and improve behavioral outcomes, demonstrating potential therapeutic value.
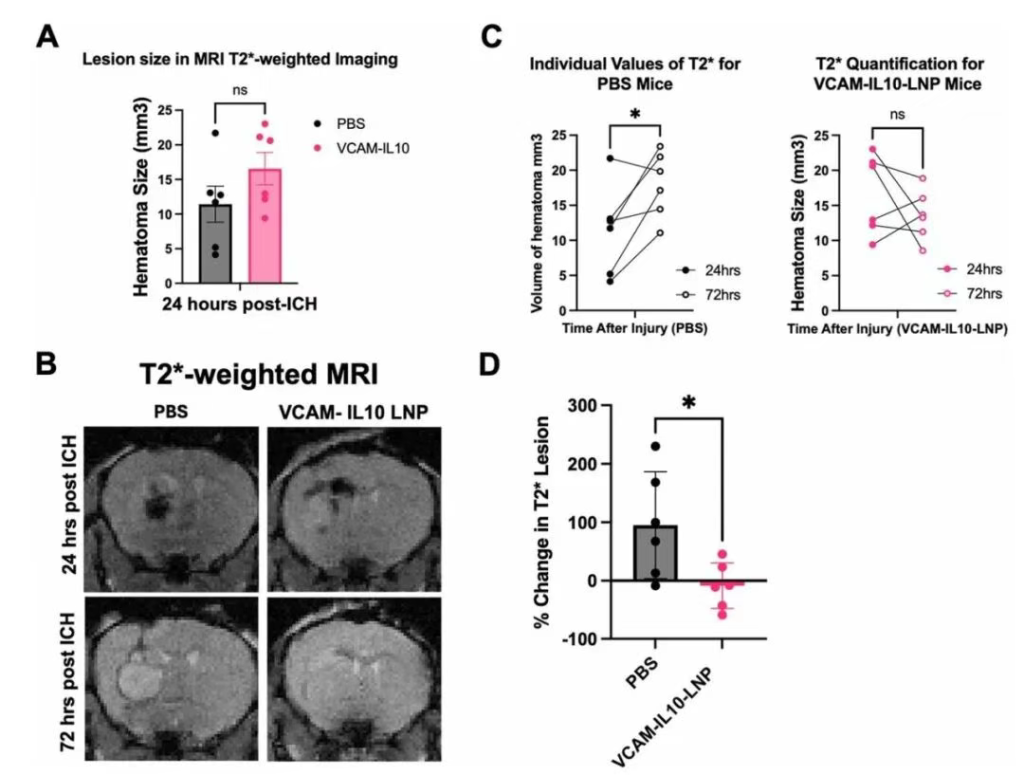
Figure 4: VCAM-IL10-LNP Treatment Correlates with Reduced Lesion Volume on MRI at 72 Hours Post-ICH
Mice received VCAM-IL10-LNPs or PBS treatment post-ICH and underwent MRI monitoring. Results: At 24 hours, there was no significant difference in hematoma size between groups, but over time, there was a significant difference in lesion volume change: the PBS group showed continuously expanding lesions on T2-weighted images, whereas the VCAM-IL10-LNP group maintained stable lesions. Notably, no significant changes in body weight or renal function were observed in any LNP-treated mice; the VCAM-mCherry-LNP control group showed elevated liver enzymes, but this was not seen in the VCAM-IL10-LNP and IgG-IL10-LNP groups.
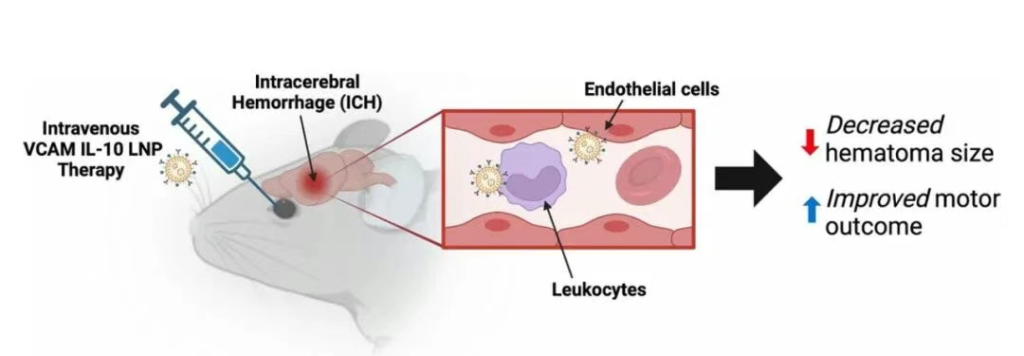
Figure 5: VCAM-LNP Delivery of IL-10 mRNA Alleviates ICH Injury
Currently, there are no effective disease-modifying therapies for ICH. The authors previously found that VCAM targeting enhanced the accumulation of therapeutic proteins in the brain. This study employed lipid nanoparticles (LNPs), which offer advantages for nucleic acid delivery, to explore whether VCAM-targeted delivery of IL-10 mRNA could more effectively improve multiple clinically relevant outcomes in ICH.
In summary, compared to non-targeted controls, VCAM-LNPs significantly increased the expression of payload mRNA in the brain in an experimental ICH model. Utilizing this strategy to deliver IL-10 mRNA effectively reduced hematoma volume and improved motor function. Nanoparticles primarily expressed in infiltrating leukocytes within the brain, predominantly macrophages. VCAM-IL10-LNPs induced a shift in macrophage phenotype, promoting an increase in anti-inflammatory/M2 subsets. This research provides new insights for treating this disease, which currently lacks effective therapies and has high mortality rates.
Source Article:
Title: Targeted lipid nanoparticles containing IL-10 mRNA improve outcomes in experimental intracerebral hemorrhage
Journal: Journal of Neuroinflammation
DOI: https://doi.org/10.1186/s12974-025-03541-0
Published: 14 October 2025
